ICS – in.vent Clinical Services
Diagnostics Studies according to IVDR 2017/746
discover our process

Ethical and regulatory clearance – Clinical validation takes longer than you think!
Prospective clinical performance studies – Worth the effort?
Careful with the terms ‘prospective’ and ‘retrospective’ – Are you referring to study design or sample procurement?
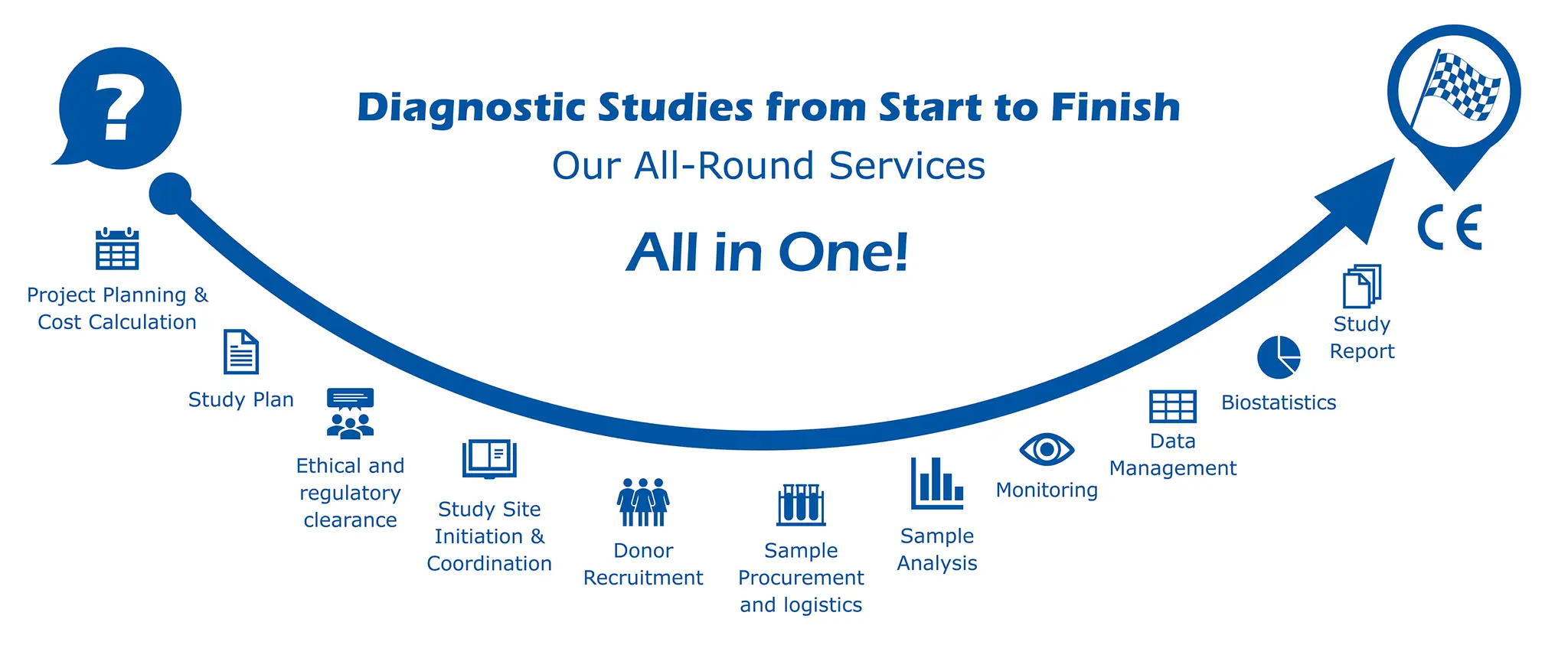
Study designs are as individual as IVD devices and their intended purposes. Every detail of study design – particularly sample size calculation – can have a huge impact on feasibility as well as price and lead time. The very first step of any validation project is therefore the precise definition of the product’s intended purpose and a corresponding study design.
IVDR and ISO 20916 express clear expectations regarding content and structure of a performance study plan. We can generate the study plan for you or you may approach us with a study plan yourself. Either way, we will take care that the submitted study plan is compliant with all applicable guidelines.
Performance studies with human specimens must be approved by ethics committees or institutional review boards (IRBs) and in some cases also a governmental authority. This requirement can be time-consuming, especially when formal errors result in additional review sessions. Learn more.
Our samples come from sources that correspond to your product’s intended purpose; as demanded by the IVDR. The sample collections we organise and monitor take place in hospitals, medical practices, blood donation centres, or nursery homes, specifically following the individual requirements of your product. Learn more.
Donor recruitment entails the generation of patient information and informed consent forms that must satisfy the good practice provisions of the national ethics boards. Being in direct contact with the study participants calls for the responsibility that participants are kept informed and that their safety and dignity is maintained throughout the study process. This is particularly the case in longitudinal studies with multiple donations from the same donors.
Suitable donors for a study can be rare, so maximum care must be given that samples maintain the highest possible quality between collection and analysis. Applying the right pre-analytical conditions at every step is key. Avoiding additional obstacles such as prolonged delay in customs during international studies requires an experienced logistics team.
Sample analysis generates the data that the whole study revolves around. The samples that have been collected for this purpose are often used up the process. Hence, any mistakes and imprecisions must be kept to an absolute minimum. The requirements for guaranteeing this differ greatly between validations of point-of-care devices and lab devices.
Monitoring is not only required by IVDR and ISO 20916 but is also an essential means of control over data quality, especially during international studies. The most well written study plan or investigator brochure may be misinterpreted by study nurses or investigators. Checking remotely or on-site whether all processes are implemented and executed as intended, is vital for a successful study project.
The generation of clinical data is the primary goal of any study, so diligent management of it is another corner stone in this process. The allocation and reproducibility of blinded sample IDs to leftover samples, source data, and reports must be guaranteed for decades of archiving.
Statistical analyses are required at the very beginning and the very end of a study. At the beginning, a thorough statistical assessment of a suitable sample size is already a decisive milestone that will be reviewed by IRBs, governmental authorities, and notified bodies. At the end of the study, biostatistics will translate the raw data points into the performance parameters that your product will be judged upon.
Our final deliverable to you will be the study report together with the raw data. Our reports are generated following guidelines and standards such as STARD 2015 and ISO 20916.
Planning, sample collection, analysis, and reporting. IVD studies from start to finish.
Diligent quality management, certified according to ISO 9001:2015 and ISO 13485:2021.
Active in all medical fields thanks to our global network of clinical partners.
20 years in the diagnostics industry. Highest familiarity with IVD and certification processes.
Implementation of all applicable standards and guidelines for guaranteed compliance.